A review of the principles and applications of various in silico tools
Scientists from Liverpool John Moores University (United Kingdom) and the Joint Research Centre (Italy) have published a review paper where they explain the underlying principles of various in silico tools and on how these are currently being applied as alternatives to animal testing in the industry (e.g. pharmaceuticals, (agro-)chemicals, personal care products, food additives) and their regulatory agencies. With this review paper they aim to provide a fundamental understanding of in silico tools to those who are unfamiliar with the topic as this knowledge can be integrated in different disciplines.
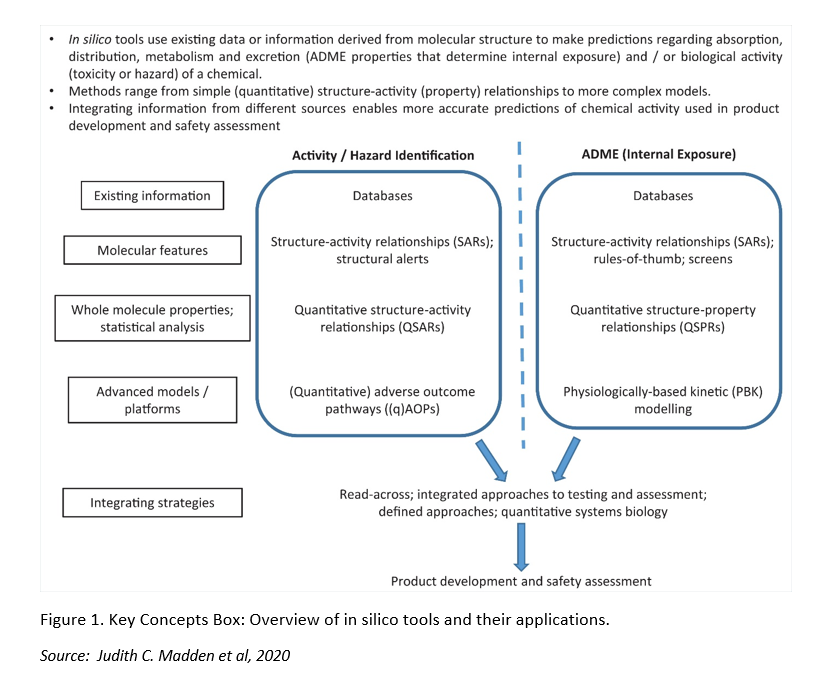
In silico tools are more frequently being used to predict the activity, toxicity and ADME properties of chemicals for the identification and optimization of drug candidates, and the safety assessment of chemicals. These in silico tools are fully based on the structure of the chemicals of interest, and make use of existing data which is stored in databases and from which information is selected to develop prediction models and algorithms. At present, in silico tools cannot be applied as one-to-one replacement methods, and so they are often combined with other techniques to provide more complementary information. Several European bodies such as the European Chemical Agency (ECHA), the European Commission’s Scientific Committee on Consumer Safety (SCCS) and the European Partnership for Alternatives to Animal Testing (EPAA) have been promoting the integration of in silico tools in testing strategies. For instance, EPAA recently organized a cross-sector partners’ forum on the use of toxicokinetics and read-across in the different regulatory domains.
In this review paper, the authors explain the principles of Structure-Activity Relationship (SARs), Quantitative Structure-Activity Relationships (QSARs), Structural Alerts (SA), read-across, physiologically-based kinetic (PBK) models, and systems biology approaches. They also provide a list of freely available databases for toxicological, physicochemical and other relevant information for safety assessment, and a summary on relevant predictive software. In silico models can also be applied in the development and mechanistic understanding of Adverse Outcome Pathways (AOPs). More in particular, as they can provide relevant information on chemical properties, they can help to predict the first interaction on biological level, i.e. the Molecular Initiating Event (MIE).
More information about the application and evaluation of these different in silico tools can be read in the full article here: https://journals.sagepub.com/doi/full/10.1177/0261192920965977?mc_cid=fe2f840e5b&mc_eid=6971491d76